- Start
- Biologiska läkemedel
- Framtida möjligheter med biologiska läkemedel
-
AAVNova –Nextgen Platform for AAV Production
AAVNova –Nextgen Platform for AAV Production
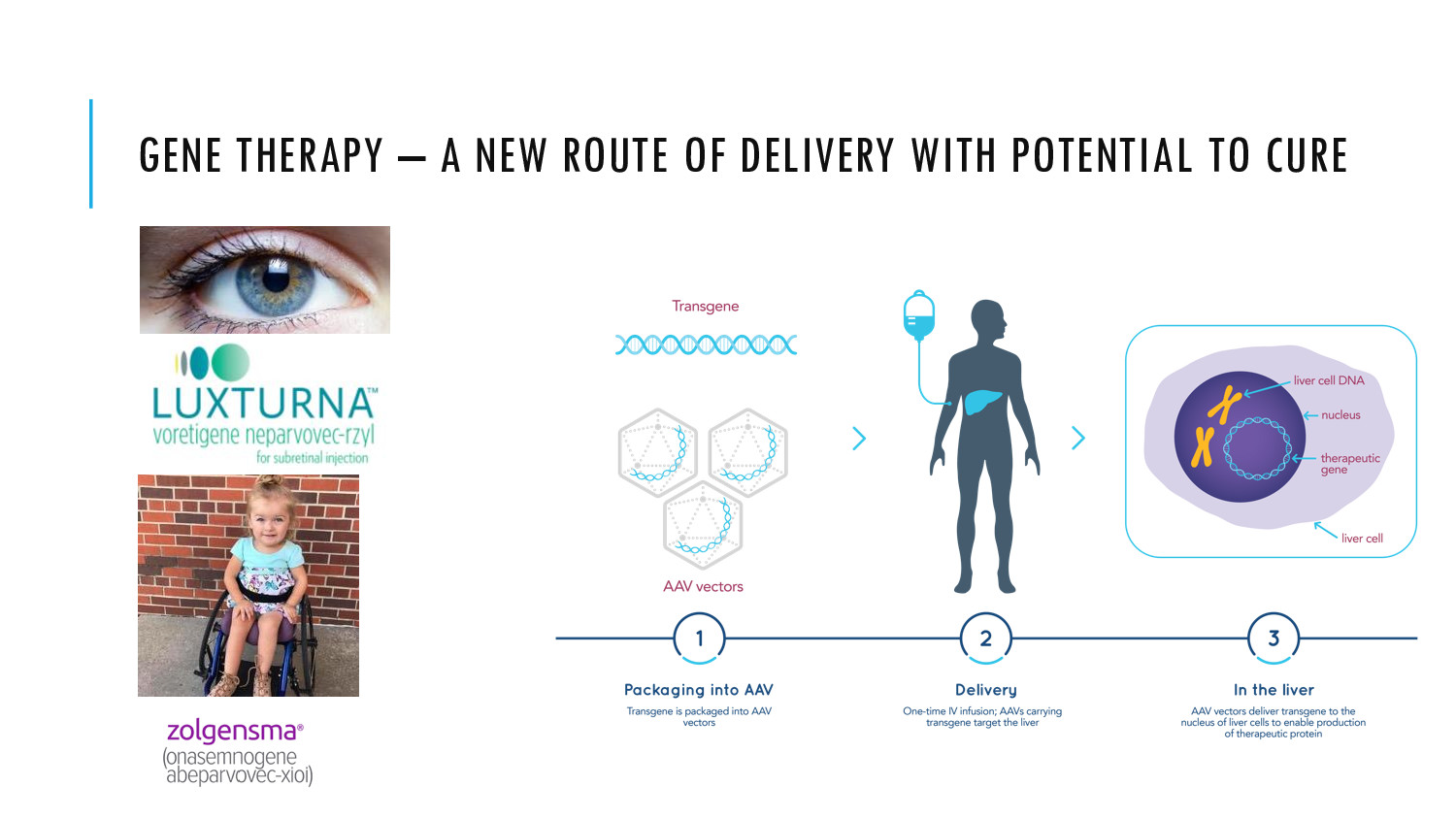
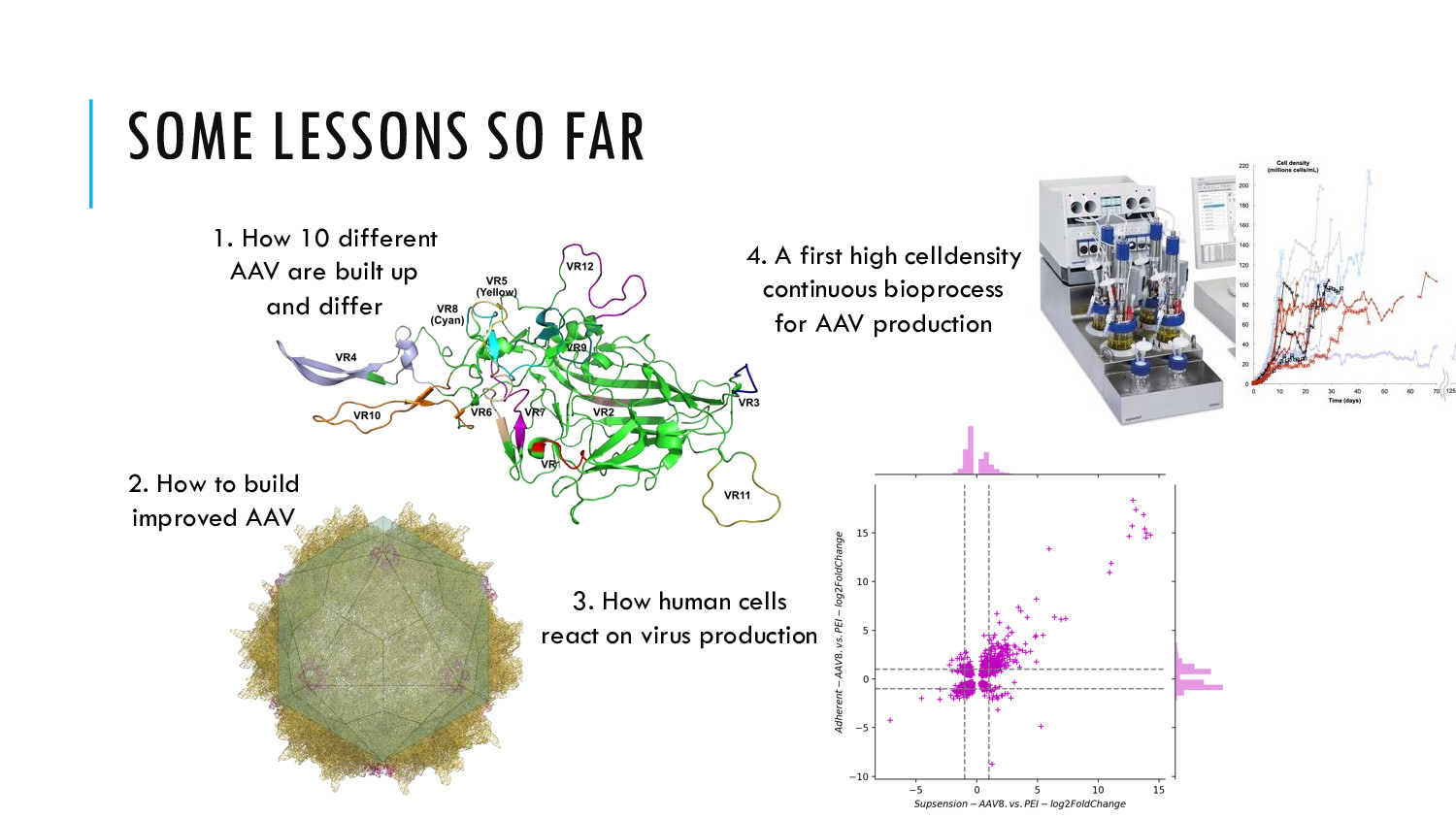
AAV Gene therapy allows for curing of previously incurable diseases. We aim to make it more accessible and scalable by improving how AAV are produced.
AAVNova




Frågor?
Senast uppdaterad 3 mars 2021
Statistik för sidan
